As Michael Douglas says, “The thing with cancer is that you want to get as early as you can.” This does not mean you should do your best to have the disease, but have the disease diagnosed early in case you have it. Cancer early detection has always been the first topic that comes to mind when we think of preventing the disease from being lethal in the first place. Statistics demonstrate that the five-year survival rate is three times higher when the cancer case is detected in the localised stage than when it is detected in the distant stage [1]. Thus, the limitations of conventional detection methods are being addressed with innovative bioliquid-based detection approaches, the challenges that such approaches face can be overcome with the utilization of biosensor technology, and recent advances in fields such bioinformatics, data science as well as artificial intelligence (AI) are catalysing the integration of all these fields to overcome the overall operating challenges in cancer early detection.
Liquid-based detection methods are considered a better option to address the deficiencies of standard detection approaches, like imaging and biopsy, but still face some challenges. First of all, imaging and biopsy techniques require highly trained personal and sophisticated infrastructure for samples collection, while bioliquid-based approaches need less trained personal for blood or no personal at all in case of saliva, urine, or stool [2, 3]. To illustrate, unlike bioliquid-based sample collection, which can be done by patient at home, medical sittings and professionals are required to take images to locate tumours or to carry out invasive biopsy protocols. Therefore, bioliquid-based approaches are key actor in the future scene of decentralised detection. Furthermore, those bioliquids contain biomarkers (Figure 1) including circulating tumour cells CTCs, circulating tumour DNA (ctDNA), RNAs, microRNA, extracellular vesicles (EVs), proteins, and metabolites representing a wide range of cancers [4]. While biopsy may fail to provide a histopathological diagnosis of a tumour due to the fact that the portion of the tumour sampled may not represent its heterogenous nature, biomarkers are accurately linked to their origin tumours leading to accurate histopathologic diagnosis [5, 6]. For instance, the use of a combination of biomarkers to detect a cancer type is seen in the example of lung cancer when it was detected with a high accuracy through a technology depending on the detection of epidermal growth factor receptor (EGFR) mutations (exon 19 deletion, and L858R mutation), and with a high sensitivity (93.75%) and selectivity (82.81%) using regression model with a combination of the five mRNA biomarkers (CCNI, EGFR, FGF19, FRS2 and GREB1) [6].
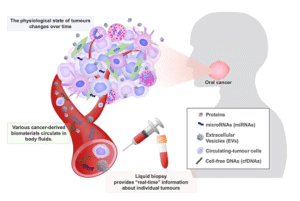
Figure 1. Liquid biopsy for oral cancer diagnosis. Various cancer-derived biomaterials are released into body fluids, such as blood, saliva, and urine [1].
The challenges bioliquid-based methods face can be addressed through biosensing technologies, paving the way for their utilization for precision and decentralised early cancer detection applications. The main challenge is that the biomarkers exist in ultralow concentrations, which goes down to femtomolar level. This issue has been addressed through highly sensitive biosensors (Tables 1, 2, and 3) [7, 8, 9]. Biosensors are generally categorized (Figure 2) with respect to the physical or chemical activity through which a biological interaction may be transformed into a signal, which can then be measured. Such a classification comprises electrochemical (Table 1), optical (Table 2), and mass-based biosensors (Table 3) among others. Each of these types is characterized by the nature of a signal they detect, such as alterations in current, light, mass, and the match to the particular biomarker and type of samples it considers. For instance, electrochemical biosensors are most efficient and sensitive to redox reactions when used to detect proteins or small molecules, whereas optical biosensors are renowned in capturing minute variations in light intensity and thus very helpful in detection of nucleic acids or exosomes. Mass-based biosensors, however, are superior at label-free mass detection, and as such would be well-suited at characterizing whole-cell or vesicle interactions. Less often used are the thermal and magnetic sensors, which find a growing interest in metabolic and multiplex applications. The basic dissimilarities between these biosensor types are also crucial to be understood during the design of systems specific to cancer diagnostics [6].
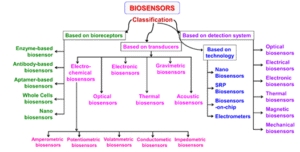
Figure 2. Classification of biosensors based on various bioreceptors and transducers used [2].
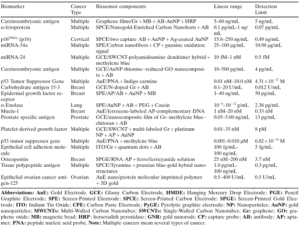
Table 1. Summary of innovative electrochemical voltametric biosensors of cancer biomarkers detection [3].
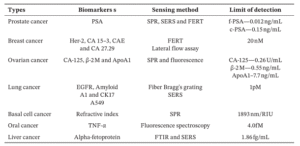
Table 2. Optical sensor methods employed for detecting different types of cancer using biomarkers present in biofluids [4].
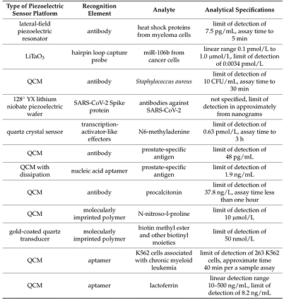
Table 3. Performance of reported piezoelectric biosensors (a type of mass-based biosensors) for cancer-related biomarkers [5].
Subtle issues arise with the strong prospects of biosensors in cancer early screening. The best way to implement the huge potential capabilities of bioliquid-based biomarkers is to detect as many biomarkers as possible to cover a wide range cancer types detection ensuring the best possible precision diagnostic. On the other hand, multiplexed sensing and diagnostic device will give massively diverse and complex readings or results. For instance, different biomarker types will be detected such as nucleic acids (ctDNAs, RNAs, and microRNAs), proteins, Extracellular vesicles (EVs) components [10]. Therefore, there is an essential need to organise and process this complex data, since we are no longer dealing with only one biomarker signal. Furthermore, such a matrix of biosensors will need an operating and automation system to guarantee that all those biosensors are functioning synergistically. Moreover, standardization is still a challenge here because of its crucial role in developing clinically trustworthy instrument able to detect a broad-spectrum of cancer types using different bioliquid samples [11].
Data science, Bioinformatics and artificial intelligence (AI) are capable to eliminate the above-mentioned constraints. Starting from the divers and complex data obtained, bioinformatic tools implement the knowledge from different fields including genomics, transcriptomics, proteomics, microbiomics, and metabolomics to process all the complex data coming from the biosensing platform. Together with bioinformatics, data science will play a fundamental role in further processing tasks such as organising the data in separate datasets and setting the stage for sophisticated analysis of the data to draw meaningful conclusions leading to accurate diagnosis. However, for accurate clinically valid diagnosis to be made, AI should be implemented to employ machine learning algorithms, predictive analysis, and multivariate statistical modelling. These applications of AI will guarantee a smoother and high-performing data processing, leading to the development of an optimal, interpretative, automatic, and integrative computational frameworks to make biosensors more reliable and adaptive to the various bioliquid composition and biomarkers combinations. In addition, data science and AI may assist in the mission of standardization through showing patterns from the big data generated by the biosensors and organised by the bioinformatic tools. In summary, Data science, Bioinformatics and artificial intelligence (AI) will contribute to the development of early cancer detection and personalized healthcare (Figure 3) [12].
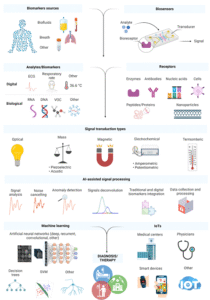
Figure 3. Schematic representation of biosensor components for detecting biomarkers. ML- and AI-based data processing enables integration and combination of traditional biomarkers with digital ones to personalize healthcare. The acquired data can then be collected, distributed, and evaluated by clinicians and individual patients [6].
References
[1]. R. L. Siegel, K. D. Miller, H. E. Fuchs, and A. Jemal, “Cancer statistics, 2022,” CA Cancer J. Clin., vol. 72, no. 1, pp. 7-33, 2022. doi.org/10.3322/caac.21708. [CrossRef]
[2] A. Altaf et al., “Utilization of an ultra-low-field, portable magnetic resonance imaging for brain tumour assessment in lower middle-income countries.,” Surgical Neurology International, vol. 14, p. 260, Jul. 2023. DOI:10.25259/SNI_123_2023. [CrossRef]
[3] W. S. El‐Deiry et al., “The current state of molecular testing in the treatment of patients with solid tumors, 2019.,” CA: a cancer journal for clinicians, vol. 69, no. 4, pp. 305–343, Jan. 2019. doi.org/10.3322/caac.21560. [CrossRef]
[4] M. Battistelli, “Liquid Biopsy: A Family of Possible Diagnostic Tools,” Diagnostics, vol. 11, no. 8, p. 1391, Jul. 2021, doi: 10.3390/diagnostics11081391. [CrossRef]
[5] R. Sinha, N. Patel, and N. Patel, “Analysing false negatives and false positives in cytopathology of breast masses along with cytohistopathological correlation,” IP Archives of Cytology and Histopathology Research, vol. 3, no. 3, pp. 144–150, Dec. 2020. httpdoi.org/10.18231/2456-9267.2018.0030. [CrossRref].
[6] X. Wang, K. E. Kaczor-Urbanowicz, and D. T. W. Wong, “Salivary biomarkers in cancer detection.,” Medical Oncology, vol. 34, no. 1, Dec. 2016, doi: 10.1007/s12032-016 0863-4. [CrossRef]
[7] S. N. Topkaya, M. Azimzadeh, and M. Ozsoz, “Electrochemical Biosensors for Cancer Biomarkers Detection: Recent Advances and Challenges,” Electroanalysis, vol. 28, no. 7, pp. 1402–1419, Feb. 2016, doi: 10.1002/elan.201501174. [CrossRef]
[8] D. N. Moorthy, D. Dhinasekaran, P. N. B. Rebecca, and A. R. Rajendran, “Optical Biosensors for Detection of Cancer Biomarkers: Current and Future Perspectives.,” Journal of biophotonics, vol. 17, no. 12, Oct. 2024, doi: 10.1002/jbio.202400243. [PubMed]
[9] M. Pohanka, “Piezoelectric Chemosensors and Biosensors in Medical Diagnostics.,” Biosensors, vol. 15, no. 3, Mar. 2025, doi: 10.3390/bios15030197. [CrossRef]
[10] J. A. Otoo and T. S. Schlappi, “REASSURED Multiplex Diagnostics: A Critical Review and Forecast,” Biosensors, vol. 12, no. 2, p. 124, Feb. 2022, doi: 10.3390/bios12020124. [CorssRef]
[11] H.-J. Baek, K.-S. Kim, M. Kwoen, E.-S. Park, H.-J. Lee, and K.-U. Park, “Saliva assay: a call for methodological standardization.,” Journal of periodontal & implant science, vol. 55, no. 1, Jan. 2024, doi: 10.5051/jpis.2304180209. [CrossRef]
[12] T. Wasilewski, W. Kamysz, and J. Gębicki, “AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring.,” Biosensors, vol. 14, no. 7, p. 356, Jul. 2024, doi: 10.3390/bios14070356. [CrossRef]
Figures
[1] Y. Naito and K. Honda, “Liquid Biopsy for Oral Cancer Diagnosis: Recent Advances and Challenges,” Journal of Personalized Medicine, vol. 13, no. 2, p. 303, Feb. 2023, doi: 10.3390/jpm13020303. [CrossRef]
[2] V. Naresh and N. Lee, “A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors.,” Sensors, vol. 21, no. 4, p. 1109, Feb. 2021, doi: 10.3390/s21041109. [PubMed]
[3] S. N. Topkaya, M. Azimzadeh, and M. Ozsoz, “Electrochemical Biosensors for Cancer Biomarkers Detection: Recent Advances and Challenges,” Electroanalysis, vol. 28, no. 7, pp. 1402–1419, Feb. 2016, doi: 10.1002/elan.201501174. [CrossRef]
[4] D. N. Moorthy, D. Dhinasekaran, P. N. B. Rebecca, and A. R. Rajendran, “Optical Biosensors for Detection of Cancer Biomarkers: Current and Future Perspectives.,” Journal of biophotonics, vol. 17, no. 12, Oct. 2024, doi: 10.1002/jbio.202400243. [PubMed]
[5] M. Pohanka, “Piezoelectric Chemosensors and Biosensors in Medical Diagnostics.,” Biosensors, vol. 15, no. 3, Mar. 2025, doi: 10.3390/bios15030197. [CrossRef]
[6] T. Wasilewski, W. Kamysz, and J. Gębicki, “AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring.,” Biosensors, vol. 14, no. 7, p. 356, Jul. 2024, doi: 10.3390/bios14070356. [CrossRef]
Denetmen: Emine ARSLAN


